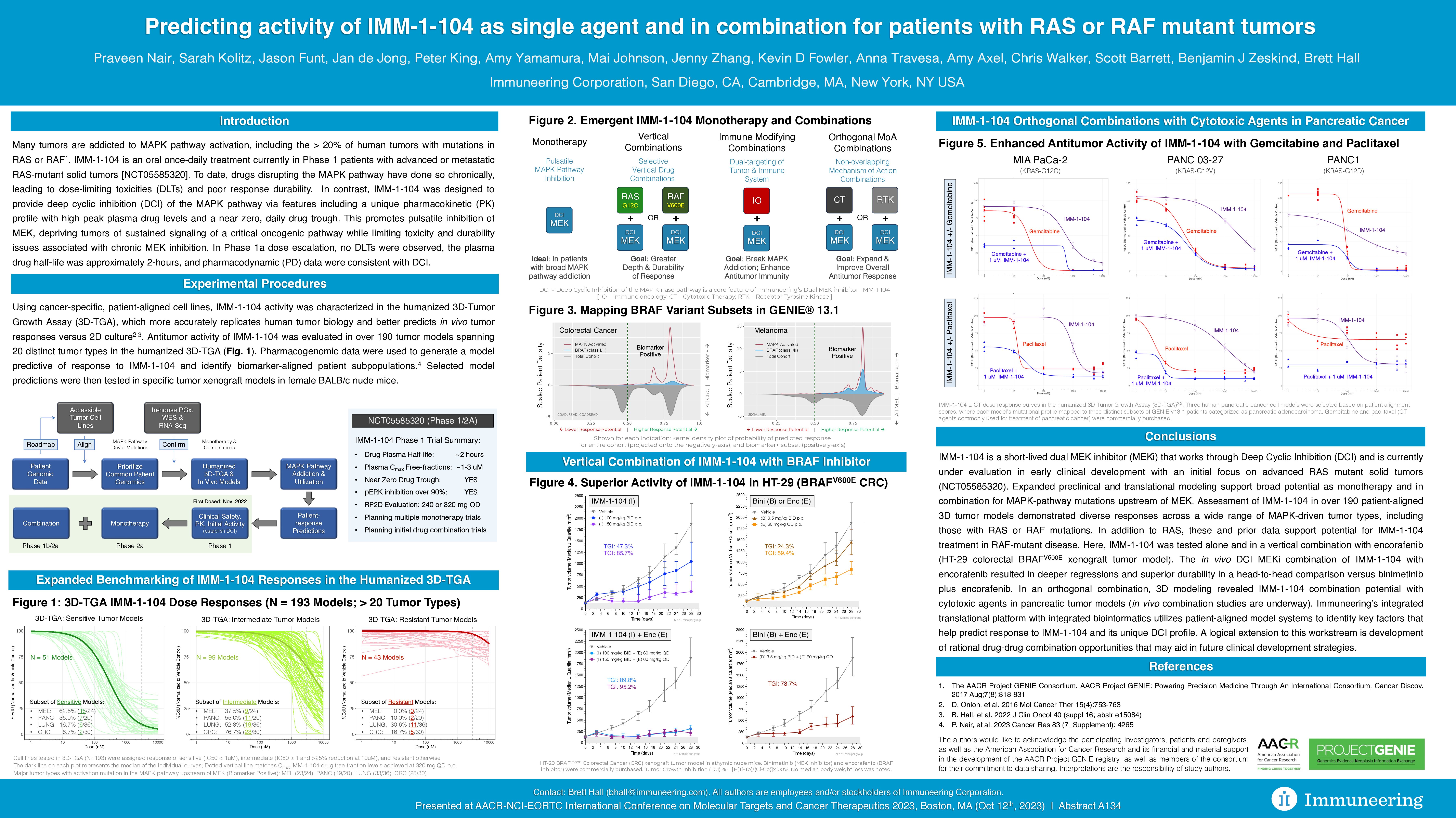
- Anti-tumor activity of IMM-1-104 was characterized in 193 tumor models spanning 20 distinct tumor types in the humanized 3D-tumor growth assay (3D-TGA) using cancer-specific, patient-aligned cell lines.
- IMM-1-104 demonstrated diverse responses across a wide range of MAPK-driven tumor types, including those with RAS or RAF mutations.
- Pharmacogenomic data were used to generate a model predictive of response to IMM-1-104 and identify biomarker-aligned patient subpopulations.
- Sensitivity to IMM-1-104 (IC50 < 1uM) tested in 3D-TGA cell lines was highest in melanoma (62.5%) followed by pancreatic cancer (35.0%) and lung cancer (16.7%).
- IMM-1-104 was tested in combination with gemcitabine or paclitaxel in humanized 3D models of pancreatic cancer, demonstrating enhanced activity and combination therapy potential.
- IMM-1-104 in combination with encorafenib drove deeper regressions and superior durability of response in a head-to-head in vivo comparison versus binimetinib plus encorafenib. Tumor growth inhibition (TGI) was between 89.8% and 95.2% with the IMM-1-104 plus encorafenib combination and 73.7% with the binimetinib plus encorafenib combination.
© 2025 Immuneering Corporation.