Our platform is enabled by our ability to efficiently analyze high-throughput molecular-level biochemical assays, including transcriptomics, genomics and/or proteomics, collectively referred to as Omics data. These different types of biochemical assays provide us with unique information about the molecular mechanisms of disease biology and drug response.
We apply our proprietary platform and approach to develop our pipeline of orally administered small molecule drug programs for the treatment of cancer. Key elements of our platform include:
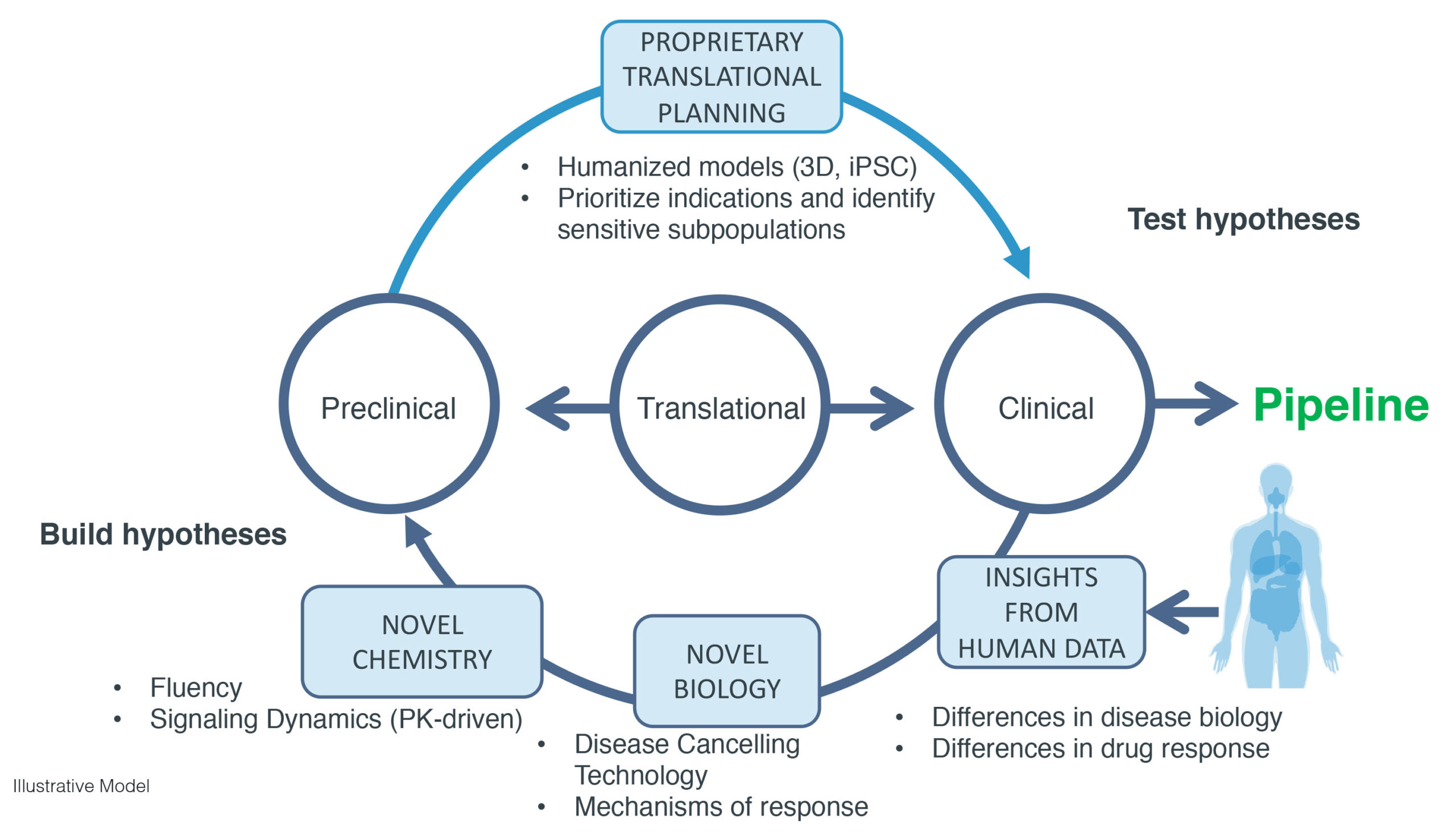
- Insights from Human Data. Compare distinct groups of individuals who differ in a certain aspect of disease or response to a particular therapy, or identify new patient subsets. We associate transcriptomic profiles to identify novel subset-specific targets.
- Novel Biology. Identify novel targets and new ways to drug existing targets using our technology and insights into mechanisms of response. Additional biologic context is derived from quantifying the extent to which different time points, concentrations and perturbations (e.g., inhibition and overexpression) may cancel a disease signal more effectively than existing drug targets.
- Novel Chemistry. Rapidly identify small molecules that selectively bind to a target of interest using our proprietary AI technology, and/or engineer PK to achieve optimal signaling dynamics. Our technology identifies the most attractive drug candidates within a library by making ranked predictions of binding affinity for all compounds, and can be run on any library containing millions of compounds.
- Proprietary Translational Planning. Use humanized preclinical models and bioinformatics to prioritize indications and identify sensitive subpopulations. Our advanced, humanized 3D-based tumor growth models are designed to more accurately predict drug response in animal models, and we believe in patients, compared to standard models. Unlike in vitro approaches, the 3D tumor growth models reflect the complexity of tumor biology given their alignment with the TME.