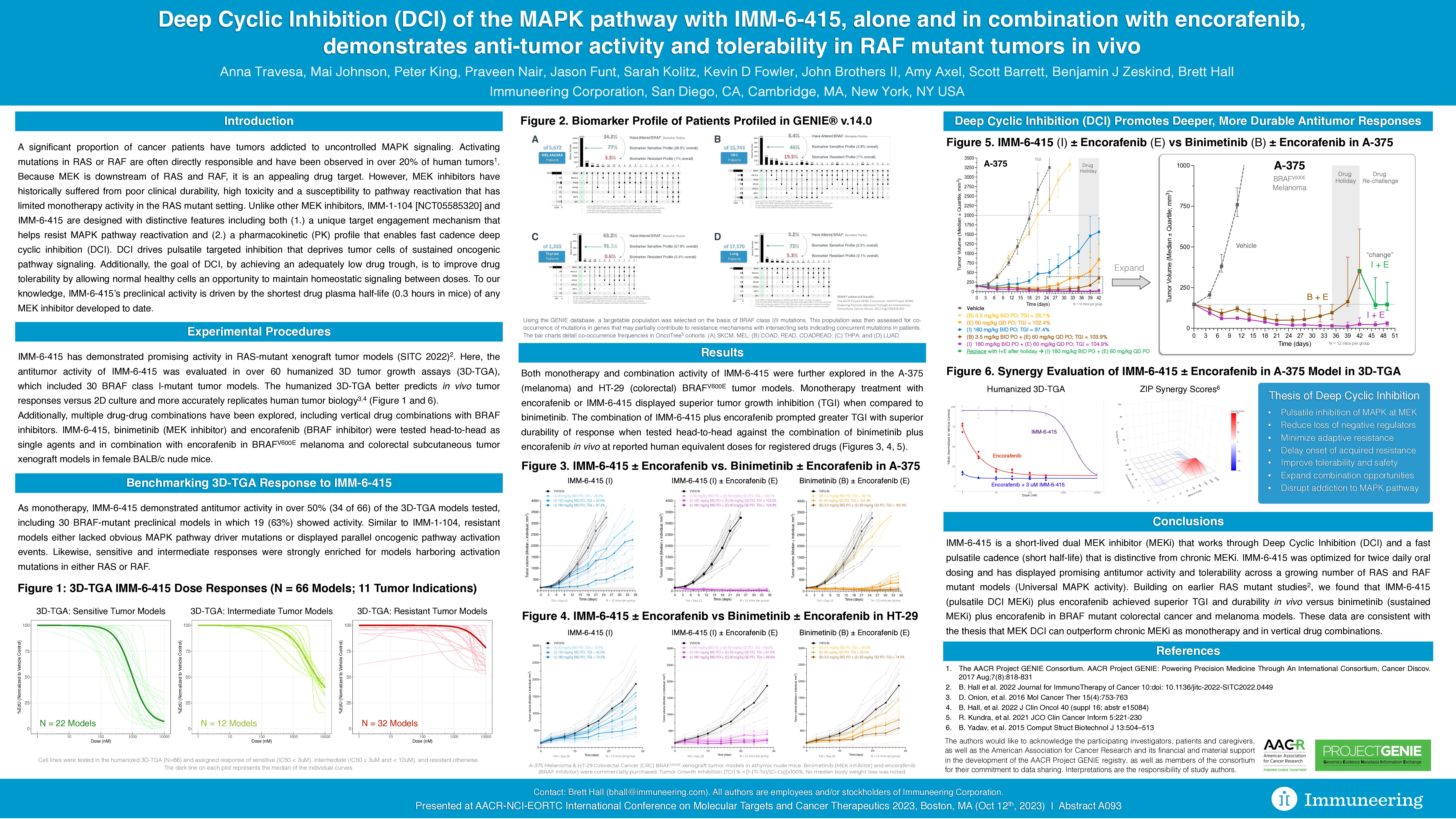
- Anti–tumor activity of IMM-6-415 was evaluated in more than 60 humanized 3D-TGA models, which included 30 BRAF class I-mutant tumor models. Multiple drug-drug combinations have been explored, including vertical drug combinations with BRAF inhibitors.
- IMM-6-415, binimetinib and encorafenib were tested head-to-head as single agents and in combination with encorafenib in BRAFV600E melanoma and colorectal subcutaneous tumor xenograft models in female BALB/c nude mice.
- As monotherapy, IMM-6-415 demonstrated anti–tumor activity in over 50% (34 of 66) of the 3D-TGA models tested, including 30 BRAF mutant preclinical models in which 19 (63%) showed activity. Similar to IMM-1-104, resistant models either lacked an obvious MAPK pathway driver mutation or displayed parallel oncogenic activation events. Sensitive and intermediate responses were also strongly enriched for models harboring an activation mutation in RAS or RAF.
- Monotherapy treatment with encorafenib or IMM-6-415 displayed superior TGI when compared to binimetinib in the A-375 (melanoma) and HT-29 (colorectal) BRAFV600E tumor models.
- In combination with encorafenib, IMM–6–415 achieved a greater TGI in vivo than the combination of encorafenib plus binimetinib in BRAFV600E colorectal cancer and melanoma tumor models, suggesting an opportunity for IMM-6-415 as monotherapy or in combination regimens for the treatment of BRAF mutant tumors.
© 2025 Immuneering Corporation.