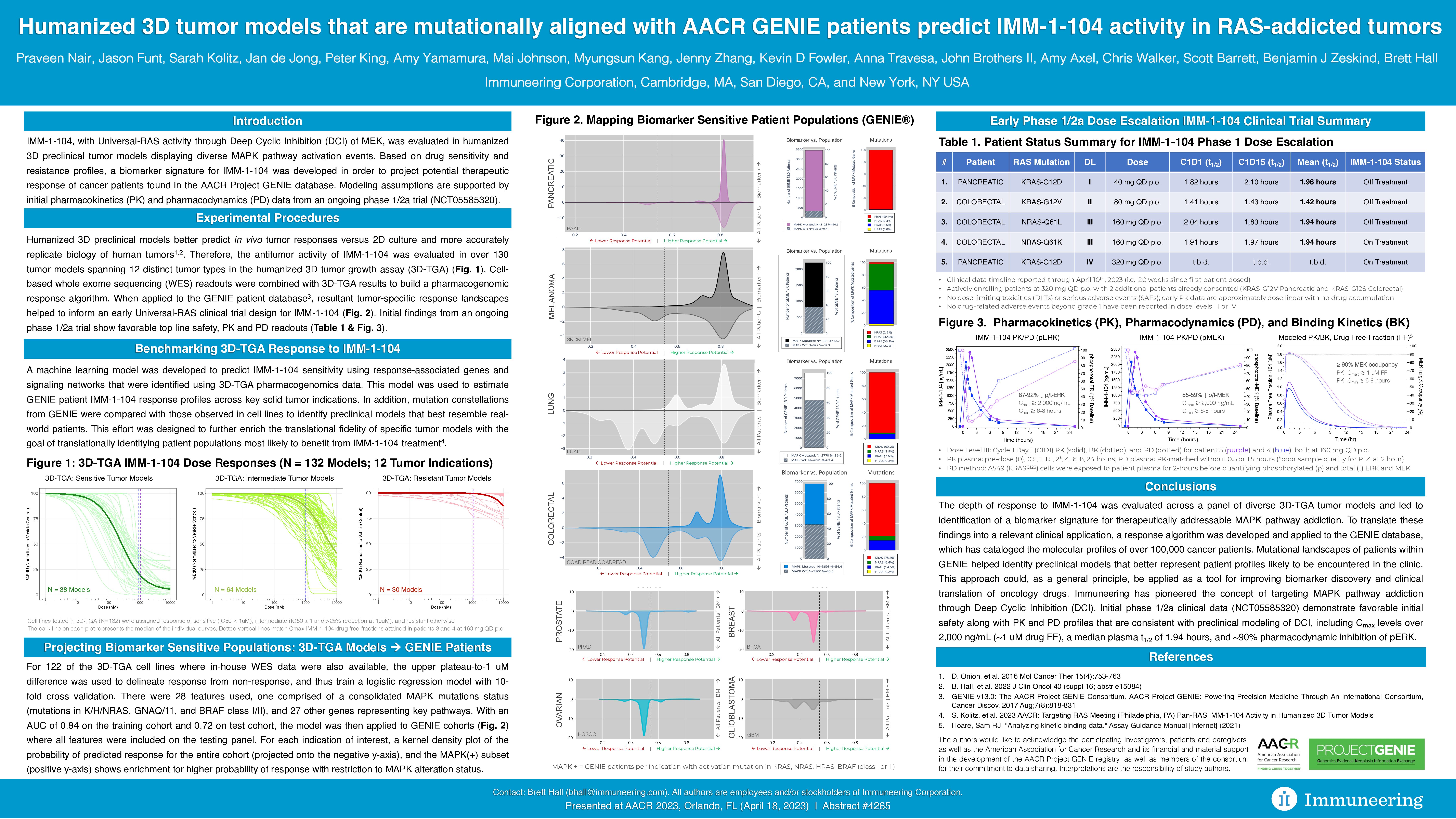
Highlights of the initial IMM-1-104 Phase 1 PK, PD and safety data presented at AACR include (as of data cut-off date of April 10, 2023, including patients with pancreatic and colon cancer):
- Significant PK Cmax levels (plasma concentration of therapy in a specific area of the body) observed with IMM-1-104 of over 2,000 ng/mL (or approximately 1 uM drug free-fraction at 160 mg once daily oral dose)
- Greater than 90 percent PD inhibition of phosphorylated extracellular signal-regulated kinase (pERK) with IMM-1-104 compared to pretreatment baseline for patients at the third dose level (160 mg once daily oral)
- A median plasma half-life (t1/2) of 1.94 hours observed with IMM-1-104 across the first three dose levels evaluable (40 mg, 80 mg and 160 mg once daily oral), in patients with pancreatic and colorectal cancer with different RAS mutations, including KRAS-G12D, the most common mutation present in pancreatic cancer
- IMM-1-104 was well tolerated with no DLTs or SAEs observed and no drug-related adverse events beyond Grade 1 observed
Title: Humanized 3D tumor models that are mutually aligned with AACR GENIE patients predict IMM-1-104 activity in RAS-addicted tumors
Date: Tuesday, April 18, 2023, 9:00am – 12:30pm ET
Poster session: AACR Project GENIE Use Cases
Abstract Number: 4265